Organisation
Structural organisation
Cooperative Association of the German Mammography Screening Program
The Cooperative Association of the German Mammography Screening Program (the so-called Kooperationsgemeinschaft Mammographie, KoopG for short) was founded in 2003 as non-profit joint venture of the National Association of Statutory Health Insurance Funds and the National Association of Statutory Health Insurance Physicians (see Legal Foundation).
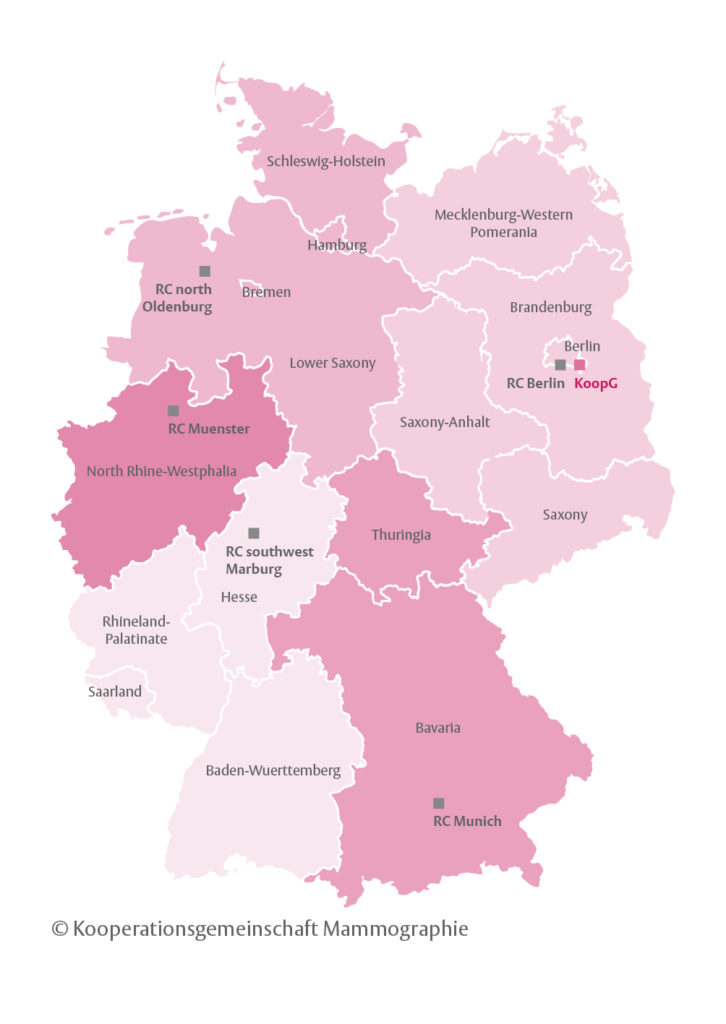
It consists of the German Mammography Screening Office in Berlin and five National Reference Centers (RC) for Mammography, located in Berlin (RC Berlin), Gießen(RC SouthWest), Münster (RC Münster), Munich (RC Munich), and Oldenburg (RC North). The Cooperative Association is responsible for quality assurance, training in the program as well as evaluations concerning quality of structure, process and results.
Major Tasks of the Cooperative Association
- Certification and regular re-certification of all screening units every 30 months
- Regular evaluation of the performance of the screening program and the quality assurance procedures
- Public relations and communication of health information related to the breast cancer screening program
German Mammography Screening Office (KoopG Office)
The KoopG Office primarily coordinates and supports the implementation of the screening directives and facilitates communication between the different structures and institutions within the program.
Major Tasks of the KoopG Office:
- Organisation and execution of certifications and re-certifications of screening units
- Preparation and publication of annual evaluation and quality reports
- Preparation of information for health professionals and women in printed form, on web pages and on social media channels
- Development and update of technical specifications for standardized documentation and evaluation
- Support of its joint partners in advancing the program and the quality assurance procedures
Reference Centers (RC)
 Five reference centers were set up in 2005 for quality management and training within the program. Each RC is headed by a medical doctor with special expertise in breast imaging and mammography screening. The directors of the RCs are obliged to head one screening unit in the region assigned to him/her. This screening unit serves as reference screening unit for training of other screening units and their staff (see Figure 1).
Five reference centers were set up in 2005 for quality management and training within the program. Each RC is headed by a medical doctor with special expertise in breast imaging and mammography screening. The directors of the RCs are obliged to head one screening unit in the region assigned to him/her. This screening unit serves as reference screening unit for training of other screening units and their staff (see Figure 1).
Major tasks of the RCs:
- Screening-specific training of physicians and radiographers
- External medical and technical quality assurance in screening units
- Providing support and advice for the lead program radiologists at the other screening units and their co-workers
- Providing medical expertise for the certification and re-certification of screening units
- Medical leadership, advisory services and support for the KoopG Office and its joint partners
In 2017, the five National RCs of the German breast cancer screening program were certified as European Reference Centers for Breast Screening, the highest certification obtainable wich conforms to EU-Guidelines.
Invitation Centers
The written invitations are issued by 14 invitation centers, which receive data from the local population registries in compliance with the data protection and registration regulations of each federal state. Rescheduling, cancellations, and acceptance of appointments are undertaken by the invitation centers upon women’s requests.
Screening Units (SU)
In Germany, 95 screening units carry out the population-wide screening for 11 million eligible women. The screening service is carried out by designated physicians only, each of them licensed for screening and/or defined assessment procedures, including histopathology, by the relevant Association of Statutory Health Insurance Physicians in each Federal State in Germany. The screening licenses are contingent upon specific qualification and training in a Reference Center (see “Quality Management” section) and upon successful certification by KoopG and the Reference Center responsible. One or two lead program radiologists are in charge of organizing breast cancer screening within the unit, including double and consensus reading, assessment of suspicious lesions with additional imaging and, if necessary, needle biopsy and multi-disciplinary screening conferences, as well as quality management for each step. The lead program radiologists cooperate with screening-licensed medical specialists for double reading as well as histopathological assessment. They may delegate stereotactic vacuum biopsies.
Each screening unit consists of at least one (mostly several) mammography units (MU) for screening and at least one assessment unit (AU) for a further assessment of any suspicious findings. In rural areas, some of the screening is is carried out in mobile mammography units. In Germany 95 screening units have been established with a total of approximately 400 sites (mammography and assessment units).
Legal Foundation
German System of Healthcare
Institutions:
In 2002, the German Bundestag decided on the introduction of an organized breast cancer screening program based on the European guidelines to ensure population-wide access, specific quality standards and the evaluation of performance and outcomes.
https://www.bundestag.de/en/parliament/function
Although the Federal Ministry of Health (BMG) provides the appropriate legislative framework [§ 25a of Book V of the German Social Code (SGB V)], it is the duty of the Federal Joint Committee to fill out this framework and to ensure that the legal instructions are implemented practically in everyday work.
https://www.bundesgesundheitsministerium.de/ministry/the-federal-ministry-of-health/?L=1
The Federal Joint Committee (Gemeinsamer Bundesaussschuss, G-BA) is a council made up of representatives from statutory health insurance funds, the German hospital federation, licensed doctors, psychotherapists and dentists. The G-BA has the authority to issue directives that are binding for healthcare providers, statutory health insurance funds and patients in Germany.
https://www.g-ba.de/english/structure/
Approximately 90 percent of the German population are insured through statutory health insurances today. The National Association of Statutory Health Insurance Funds (NASHIF) is the central association of the health insurance funds at federal level. The tasks of the NASHIF range from negotiating on contracts and remuneration agreements for medical and dental treatment, providing data for the risk structure compensation scheme, which determines the distribution of finances by the health funds, through to shaping health care directives in the G-BA.
https://www.gkv-spitzenverband.de/english/english.jsp
https://www.gkv-spitzenverband.de/media/dokumente/presse/publikationen/Imagebroschuere_GKV-Spitzenverband_Einzelseiten_Englisch_2012.pdf
The National Association of Statutory Health Insurance Physicians (NASHIP) represents the political interests of all office-based physicians and psychotherapists. It represents the doctors’ positions in legislative processes e.g. in the G-BA, maintains the federal registry of physicians, and concludes contracts with the national confederations of the health insurance funds and other parties in the healthcare sector.
http://www.kbv.de/html/about_us.php
Screening Directives:
The Early Cancer Detection Directive (KFE-RL) issued by the G-BA, is the regulatory framework for the medical and organizational structure of the German breast cancer screening program. The KFE-RL thereby defines fundamental procedures such as the invitation to and information for women, the screening process (including further assessment) as well as the nationwide evaluation of the screening program.
Full text of the KFE-RL (German only):
https://www.g-ba.de/richtlinien/17/
The Federal Collective Agreement for Medical Practitioners (Bundesmantelvertrag-Ärzte, BMV-Ä) is a contract between the NASHIP and the NASHIF and sets quality standards for contracted physicians. Annex 9.2 of the BMV-Ä defines the quality standards for the medical care within the breast cancer screening program. This includes precise specifications regarding the qualifications and training of screening personnel, physico-technical quality control procedures for the imaging devices, a comprehensive quality management system for the screening service as well as the regular certification of the screening units by the KoopG.
Full text of the BMV-Ä (German only):
http://www.kbv.de/media/sp/09.2_Mammographie.pdf
Federalism:
Germany is composed of 16 federal states. The healthcare system, population and cancer registries, as well as privacy and data protection authorities are the responsibility of each individual state. When the screening program was introduced, state legislation had to be adapted to provide the necessary legal foundation to carry out screening service including quality management and evaluation, in accordance with the federal screening directives.
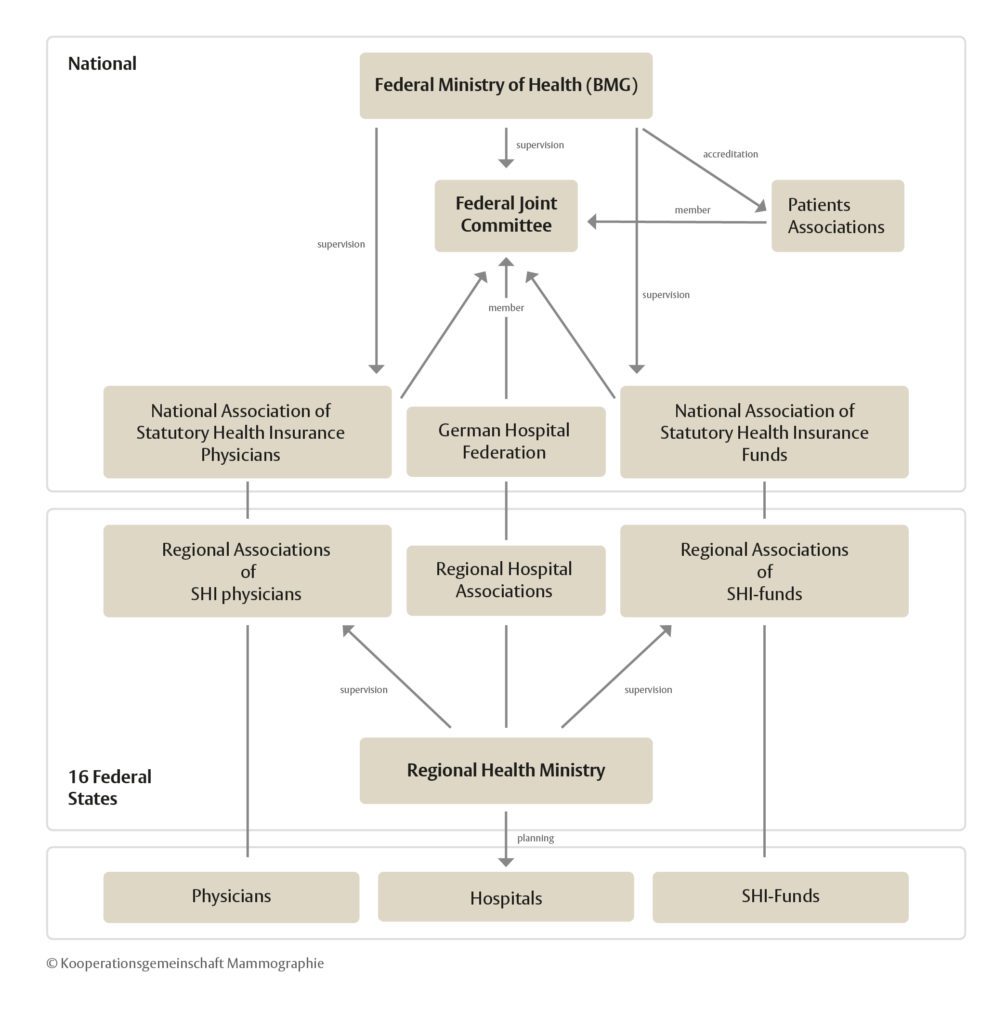
Figure 2: German system of health care
Scientific Advisory Board
Scientific Advisory Board
A scientific advisory board is appointed by the joint partners´ board meeting to provide independent scientific expertise. The members of the board are medical specialists in various fields related to breast cancer screening, diagnosis, therapy, and epidemiology. The advisory board meets regularly twice a year to advise and support KoopG and its joint partners.
Mayor tasks:
- Monitoring the quality reports and program evaluation reports
- Consultation in quality management and evaluation, particularly regarding international requirements
- Advice on further development of the system
- evaluation of current literature including drafting of expert’s reports if needed
- definition of research evaluating the effects of mammography screening
Members of the scientific board:
Prof. Alexander Katalinic
Institute for Social Medicine and Epidemiology, University of Lübeck and Registry office cancer registry Schleswig-Holstein
Ratzeburger Allee 160
23538 Lübeck
Prof. Tanja Fehm
University Women’s Clinic on University Hospital Düsseldorf
Moorenstr. 5
40225 Düsseldorf
Prof. Annette Lebeau
Institute for Pathology
University Medical Center Hamburg-Eppendorf
Martinistraße 52
20251 Hamburg
Prof. Markus Müller-Schimpfle
Clinic for Radiology, Neuroradiology & Nuclear Medicine
Klinikum Frankfurt Höchst GmbH
Gotenstraße 6-8
65929 Frankfurt a. M. – Höchst
Prof. Dr. med. Solveig Kristin Roth Hoff
Oslo University hospital Ullevaal
Norway
Prof. Andreas Stang, MPH
Center for Clinical Epidemiology (ZKE)
c / o Institute for Medical Informatics, Biometrics &
Epidemiology (IMIBE), Essen University Hospital
Colonia Haus
Zweigertstr. 37
45130 Essen